Given the following chemical equilibria, N2(g) + O2(g) 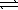 2 NO(g)
2 NO(g)
K1
N2(g) + 3 H2(g) 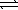 2 NH3(g)
2 NH3(g)
K2
H2(g) + 1/2 O2(g) 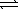 H2O(g)
H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g) + 5 O2(g) 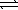 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
K c
Definitions:
Sympathetic
Showing compassion, understanding, or concern towards others, often in situations where they are in distress.
Somatic
Somatic relates to the physical body as opposed to the mind, often used in contexts discussing bodily disorders without organic or physiological causes.
Autonomic
Relating to the autonomic nervous system, which controls involuntary body functions such as heartbeat and digestion.
Parasympathetic
The branch of the autonomic nervous system responsible for regulating the body's rest and digest functions, opposing the sympathetic system.
Q3: Given the equilibrium constants for the following
Q9: Calculate the value of the equilibrium constant
Q15: Elementary steps in a reaction mechanism often
Q23: Freon-113,C<sub>2</sub>Cl<sub>3</sub>F<sub>3</sub>,has an enthalpy of vaporization of 27.0
Q26: In which of the following reactions is
Q30: Which of the following cannot be used
Q31: Which of the following is the correct
Q54: Ammonia reacts with oxygen and water to
Q57: For the formation of 1 mol of
Q67: When you draw the Lewis structure for