An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq) and 0.120 M NH3(aq) .At equilibrium,the C6H5O-(aq) concentration is 0.050 M.Calculate the equilibrium constant,K,for the reaction below. C6H5OH(aq) + NH3(aq) 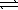 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)
Definitions:
Territory
A defined geographical area under the jurisdiction of a political entity or state.
Soldier Deaths
The fatalities of military personnel during active duty, often examined in the context of specific conflicts or wars to analyze their impact and consequences.
Prisoner-of-war Camps
Facilities where prisoners of war (POWs) are held during conflict, intended to detain enemy combatants away from the battlefield while adhering to certain treatment standards set by international law.
Disease
Any condition that impairs normal physiological function, often characterized by specific symptoms and signs and affecting part or all of an organism.
Q11: A gas sample is heated from −20.0°C
Q30: Calculate the pH of a solution made
Q32: If K<sub>c</sub> = 0.124 for A<sub>2</sub> +
Q36: Above its critical temperature and pressure,a substance
Q37: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar mass
Q45: Which of the following samples contains the
Q46: Which of the following is true of
Q52: Which of the following relationships is not
Q55: Below is a proposed mechanism for the
Q64: For a certain overall second-order reaction with