The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows. N2(g) + 3 H2(g) 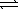 2 NH3(g)
2 NH3(g)
ΔH = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
Definitions:
Quality Assurance
A systematic process designed to determine if a product or service meets specified requirements.
Capital Acquisitions
The process of acquiring fixed, long-term assets that are intended to be used in the production of goods or services for generating future revenue.
Information Technology
The use of computers, storage, networking, and other physical devices, infrastructure, and processes to create, process, store, secure, and exchange all forms of electronic data.
Functionality
The range of operations that can be performed by a product or a system, especially in terms of its practical use and performance capability.
Q4: Which of the following linear chain alcohols
Q14: Which acid-base reaction results in acidic solution?<br>A)
Q15: The bandgap of ZnTe is 218 kJ/mol.What
Q20: How many moles of solid NaF would
Q23: The mechanism of the chlorine atom catalyzed
Q37: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg" alt="The Arrhenius
Q42: Aqueous hydrochloric acid reacts with magnesium to
Q46: Magnesium sulfide (molar mass 56.37 g/mol)has a
Q80: When a Lewis acid and a Lewis
Q84: It is possible to make a barometer