Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 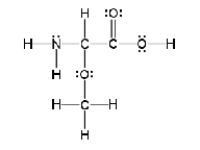
Definitions:
Related Questions
Q7: What is the equilibrium partial pressure of
Q33: The thermochemical equation for the combustion of
Q35: The ideal gas law can be modified
Q49: Non-ideal behavior for a gas is most
Q53: What mass of Zn(NO<sub>3</sub>)<sub>2</sub> must be diluted
Q62: A 2.288 g sample of a hydrocarbon
Q66: London dispersion forces are the only significant
Q67: List all the intermolecular forces present in
Q68: The change in energy for which of
Q95: Given the following acid dissociation constants: K<sub>a</sub>