Given the following acid dissociation constants: Ka (H3PO4) = 7.5 × 10-3
Ka (NH4+) = 5.6 × 10-10
Determine the equilibrium constant for the reaction below at 25 °C.
H3PO4(aq) + NH3(aq) 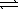 NH4+(aq) + H2PO4−(aq)
NH4+(aq) + H2PO4−(aq)
Definitions:
Demand Elastic
Referring to a situation where the quantity demanded of a good or service significantly changes in response to a change in its price.
Price-Elasticity Coefficient
A numeric value that measures how much the quantity demanded of a good responds to a change in the price of that good, indicative of its price sensitivity.
Price Reduction
A decrease in the selling price of goods or services, often intended to stimulate demand or respond to competitive market pressures.
Price Elasticity
The measure of how responsive the quantity demanded or supplied of a good or service is to a change in its price.
Q10: If a reaction is second-order with respect
Q11: Calculate Δ<sub>r</sub>G° at 25.0 °C for the
Q30: Colloids are described by all of the
Q34: What is the distance,in atomic radii,along any
Q39: If Δ<sub>r</sub>G° > 0 for a reaction
Q47: The concentration of Pb<sup>2+</sup> in an aqueous
Q57: For the reaction Br<sub>2</sub>(l)→ 2Br(g),_.<br>A) ΔH is
Q60: Calculate the copper(II)ion concentration at 25 °C
Q60: Given the following reactions, AgBr(s) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7480/.jpg"
Q62: What is the outer shell ground state