Combustion of 2.14 g of liquid benzene (C6H6) causes a temperature rise of 16.2 °C in a constant-pressure calorimeter that has a heat capacity of 5.53 kJ/°C.What is ΔH for the following reaction? C6H6(l) + 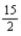 O2(g) → 6 CO2(g) + 3 H2O(
O2(g) → 6 CO2(g) + 3 H2O(  )
)
Definitions:
Sell Order
An instruction to a broker to sell a specified quantity of a security at the current market price or at a specific price.
Portfolio Value
The total worth of all the financial assets held by an individual or institution.
Heart Attack
A medical emergency characterized by the death of heart muscle due to the loss of blood supply.
Quarterly Dividend
A distribution of earnings to shareholders, done four times a year, each quarter, by a corporation.
Q2: The compound P<sub>4</sub>S<sub>3</sub> is used in matches.It
Q4: What directory service protocol is used by
Q6: According to the Bohr model of the
Q10: For which of the following molecules or
Q20: Which of the following thermodynamic quantities are
Q29: The contribution for which de Broglie is
Q35: Refer to diagram 9-1.Identify the molecule with
Q41: Which of the following compounds has a
Q58: A particular gas exerts a pressure of
Q66: Which species in the reaction below undergoes