Determine the enthalpy change for the decomposition of calcium carbonate CaCO3(s) → CaO(s) + CO2(g)
Given the thermochemical equations below.
Ca(OH) 2(s) → CaO(s) + H2O( 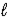 )
)
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH) 2(s) + CO2(g) → CaCO3(s) + H2O( 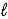 )
)
ΔrH° = −113.8 kJ/mol-rxn
C(s) + O2(g) → CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s) + O2(g) → 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
Definitions:
Project Quality Manager
An individual responsible for ensuring that the project's outputs, processes, and systems meet the required quality standards.
Client's Product
A good or service that is produced or provided specifically to meet the needs or requirements of a client.
Darnall-Preston Complexity Index
A framework used to assess the complexity of managing projects, based on dimensions such as size, diversity, and uncertainty.
Complexity
The state or quality of being intricate or complicated, often making analysis, management, or understanding challenging.
Q4: can be divided in up to four
Q9: Which element has the following ground state
Q11: Which one of the following statements is
Q15: What is the formal charge of the
Q19: Which of the following intermolecular forces or
Q24: Assuming a large number of measurements is
Q48: When 1 mole of Fe<sub>2</sub>O<sub>3</sub>(s)reacts with H<sub>2</sub>(g)to
Q58: The energy associated with a stretched spring
Q62: Which of the following is a correct
Q64: Express 0.00580 in exponential notation.<br>A) 5.80 ×