Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below. Ca(OH) 2(s) → CaO(s) + H2O( 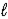 )
)
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH) 2(s) + CO2(g) → CaCO3(s) + H2O( 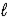 )
)
ΔrH° = −113.8 kJ/mol-rxn
C(s) + O2(g) → CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s) + O2(g) → 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
Definitions:
Industry Entry
The process of a new competitor or company beginning operations in a particular market.
Economic Profit
Economic profit is the surplus obtained after subtracting both the explicit and implicit costs from total revenues, emphasizing the opportunity costs of resources used.
Economic Loss
A decrease in monetary value, wealth, or resources, especially as a result of business activities or market factors.
Industry Supply Curve
A graphical representation that shows the relationship between the price of a good and the total output of the industry for that good.
Q11: A particular compound has an enthalpy of
Q16: At its boiling point of 58.8 °C,2.82
Q23: Freon-113,C<sub>2</sub>Cl<sub>3</sub>F<sub>3</sub>,has an enthalpy of vaporization of 27.0
Q23: Which of the following sets of ions
Q30: Which of the following atoms of elements
Q39: Which of the following is NOT a
Q41: Round 0.00274485 to 1 significant figure.<br>A) 0.00<br>B)
Q58: Which of the following compounds is insoluble
Q60: Place the following atoms in order of
Q69: Write a balanced chemical equation for the