The thermochemical equation for the combustion of methanol is given below. CH3OH( 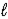 ) + 3/2 O2(g) → CO2(g) + 2 H 2O(g)
) + 3/2 O2(g) → CO2(g) + 2 H 2O(g)
ΔrH° = -638.7 kJ/mol-rxn
What is the enthalpy change for the combustion of 8.59 g of CH3OH?
Definitions:
Debt Ratio
A financial ratio that measures the extent of a company's leverage, calculated by dividing total liabilities by total assets.
Profit Margin
Profit margin is a financial metric that measures the percentage of profit a company retains after subtracting its costs from its revenue, reflecting the overall profitability of the business.
Equity Multiplier
A financial ratio indicating how much of a company's assets are financed by stockholder's equity, illustrating the degree of financial leverage used.
Times-Interest-Earned (TIE) Ratio
Determined by dividing earnings before interest and taxes by the interest charges. This ratio measures the extent to which operating income can decline before the firm is unable to meet its annual interest costs.
Q3: Which of following would be classified as
Q9: Which one of the following substances is
Q14: What is the name of the element
Q25: Determine the standard enthalpy of formation of
Q40: The elements of group 5A,the nitrogen family,form
Q43: If a cordless phone operates at a
Q43: Normal boiling point is defined as<br>A) the
Q57: The boiling point of liquid argon is
Q61: Which of the following is the correct
Q78: Write the names of metalloids present in