Determine the standard enthalpy of formation of calcium carbonate from the thermochemical equations given below. Ca(OH) 2(s) → CaO(s) + H2O( 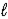 )
)
ΔrH° = 65.2 kJ/mol-rxn
Ca(OH) 2(s) + CO2(g) → CaCO3(s) + H2O( 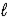 )
)
ΔrH° = −113.8 kJ/mol-rxn
C(s) + O2(g) → CO2(g)
ΔrH° = −393.5 kJ/mol-rxn
2 Ca(s) + O2(g) → 2 CaO(s)
ΔrH° = −1270.2 kJ/mol-rxn
Definitions:
Depreciation Expense
The portion of the cost of a fixed asset deducted as an expense over its useful life, reflecting the asset's consumption or wear and tear.
Common Stock
Shares of ownership in a corporation, granting holders voting rights and a share in the company's profits through dividends.
Long-term Debt
Debt that is not due within the next year and is often used to purchase or invest in significant assets or finance ongoing operations.
Q5: You can identify a metal by carefully
Q13: Which of the following virtualization products uses
Q14: What are the chunks called into which
Q17: If 46.1 g of copper at 11.6
Q23: The command-line method for creating users in
Q37: What storage solution involves a third-party company
Q40: What type of disk is required if
Q55: How much heat is liberated at constant
Q67: Sulfur trioxide (SO<sub>3</sub>)is produced from the oxidation
Q74: If two or more species have the