What is the pH of a buffer solution that is 0.295 mol L-1 in hypochlorous acid (HClO) and 0.373 mol L-1 in sodium hypochlorite (NaClO) ? The 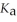 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 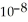 .
.
Definitions:
Investment
This refers to the allocation of resources, often financial, in assets or projects with an expectation of generating future returns.
Lower Partial Standard Deviation
A measure of the risk of negative asset returns, focusing only on the volatility of returns that fall below the average.
Extremely Negative Returns
Refers to significantly below-average returns on investments, often resulting in substantial losses.
Value At Risk
A financial metric used to estimate the potential loss in value of a portfolio over a defined period for a given confidence interval.
Q16: Determine Δ<sub>r</sub>G° at 298 K using the
Q22: What is meant by the term, "structural
Q77: Identify the missing particle in the following
Q94: Express the equilibrium constant for the following
Q98: What is the hydronium ion concentration of
Q107: Which one of the following salts, when
Q114: Calculate the pH of a solution formed
Q116: pH = pK<sub>a</sub><br>A)equivalence point of a weak
Q137: Give the name of the compound that
Q139: Which of the following acids (listed with