What is the pH of a solution made by mixing 10.00 mL of 0.10 mol L-1 acetic acid with 10.00 mL of 0.10 mol L-1 KOH? Assume that the volumes of the solutions are additive. Ka = 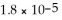 for CH3COOH.
for CH3COOH.
Definitions:
Court Ordered Decree
A formal order issued by a court that mandates or legally finalizes a decision, often related to divorce, child custody, or support.
Married Filing Separately
This is a financial status for spouses permitting them to file their taxes individually by reporting their own income, deductions, and exemptions on different tax returns.
Student Loan Interest
Interest paid on a loan taken out to fund educational expenses, which may be deductible under certain conditions for tax purposes.
Eligible Taxpayer
Eligible Taxpayer is a term defining someone who meets specific criteria set by tax laws to benefit from certain deductions, credits, or tax advantages.
Q6: What is the shorthand notation that represents
Q7: Determine the identity of the daughter nuclide
Q18: Use the following thermodynamic values to calculate
Q24: Bronsted-Lowry base<br>A)electron pair donor<br>B)produces protons in aqueous
Q37: The standard emf for the cell using
Q95: A voltaic cell is constructed with two
Q108: Which of the following is the correct
Q128: Determine Δ<sub>r</sub>G° at 298 K using the
Q148: Consider the following reaction at equilibrium. What
Q154: Calculate the pH of a 0. 080