What is the pH of a buffer solution that is 0.251 mol L-1 in lactic acid and 0.151 mol L-1 in sodium lactate? The 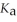 of lactic acid is 1.4 ×
of lactic acid is 1.4 × 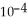 .
.
Definitions:
Borrowings
Funds that a company or individual takes on loan from another party, to be repaid over time with interest.
Denominated
To specify the currency in which a financial transaction or instrument is expressed or required to be settled.
Exchange Rate
The price of one country's currency expressed in the currency of another country.
Q22: Which of the following statements is TRUE?<br>A)
Q40: Express the equilibrium constant for the following
Q77: Which of the following represents the integrated
Q96: The first-order rearrangement of CH<sub>3</sub>NC is measured
Q101: What is the activation energy for a
Q101: Based on the following information, Cl<sub>2</sub>(g) +
Q124: Determine the equilibrium constant for the following
Q130: Phosphorus and chlorine gases combine to produce
Q140: A 100.0 mL sample of 0.18 mol
Q146: Which of the following statements is TRUE