Using the following equation for the combustion of octane, calculate the amount of moles of oxygen that reacts with 100.0 g of octane. The molar mass of octane is 114.33 g 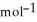 . The molar mass of carbon dioxide is 44.0095 g
. The molar mass of carbon dioxide is 44.0095 g 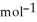 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 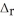 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Mixed Sales
Transactions that involve both the provision of a service and a tangible product, where different aspects of the sale may be subject to varying legal rules.
Combined Sales
The total sales volume resulting from the aggregation of different products or services sold by a company.
Contracts
Legally binding agreements between two or more parties that are enforceable by law.
Tangible Things
Objects or items that can be physically touched and seen, possessing material existence as opposed to abstract concepts.
Q1: Give the ground-state electron configuration for Cd<sup>+2</sup>.
Q31: How many valence electrons does an atom
Q43: According to the following thermochemical equation, what
Q54: Identify what a coffee cup calorimeter measures.<br>A)
Q64: List the noble gas that has the
Q74: Which of the following gas samples would
Q104: Use the periodic table to predict the
Q124: Draw the best Lewis structure for CH<sub>3</sub><sup>+</sup>.
Q125: An element that has the outer electron
Q203: A solution is prepared by adding 1.40