According to the following thermochemical equation, what mass of H2O (in g) must form to produce 975 kJ of energy? SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(l) 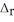 H° = -184 kJ
H° = -184 kJ
Definitions:
Depression
A psychological condition marked by a consistently low mood or a lack of enthusiasm for activities, significantly disrupting everyday functioning.
Dementia
A broad category of brain diseases that cause a long-term and often gradual decrease in the ability to think and remember, significantly affecting daily functioning.
Geographic Closeness
Refers to the physical proximity between places or people, affecting the ease of travel, communication, and relationship maintenance.
Perception
The process by which individuals interpret and organize sensory information to understand their environment.
Q2: Given w = 0, an endothermic reaction
Q27: What volume will a balloon occupy at
Q40: Use the bond energies provided to estimate
Q49: Using the following equation for the combustion
Q79: At what temperature does argon have a
Q84: Place the following gases in order of
Q84: Define ionization energy.
Q98: Choose the paramagnetic species from below.<br>A) Ca<br>B)
Q131: Place the following elements in order of
Q227: How many moles of LiI are contained