Using the following equation for the combustion of octane, calculate the heat of reaction for 100.0 g of octane. The molar mass of octane is 114.33 g 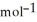 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 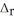 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Safety Policies
Official documents outlining the procedures and guidelines to ensure the physical and psychological well-being of employees in the workplace.
Scenario Planning
A strategic planning method that organizations use to envision and prepare for various future conditions or events.
Professional Consultants
Expert individuals or firms that provide specialized advice and services to businesses to improve their operations, strategies, or profitability.
Strategic Indicators
Key performance indicators (KPIs) used to assess the success of an organization's strategic objectives.
Q10: According to the following reaction, what amount
Q15: How many lone pairs of electrons are
Q18: What are the required coefficients to properly
Q40: Which of the following statements is TRUE?<br>A)
Q81: Identify the percent yield when 28.16 g
Q87: How many of the following elements have
Q113: Identify the compound with the highest magnitude
Q115: Identify the net ionic equation for the
Q152: A 2.38 g sample of phenol (C<sub>6</sub>H<sub>6</sub>O,
Q160: For a process at constant pressure, 62,000