How much energy is evolved during the reaction of 48.7 g of Al according to the reaction below? Assume that there is excess
Fe2O3. Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 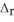 H° = -852 kJ
H° = -852 kJ
Definitions:
Market Share
The percentage of total sales volume in a market captured by a brand or company.
Industry Trends
Patterns and general directions in which a specific industry is moving, influenced by new technologies, customer behaviors, and market demands.
Evaluation Phase
The stage in a product or project lifecycle where its performance, effectiveness, or value is assessed.
Strategic Marketing Process
An approach to creating, implementing, and evaluating marketing strategies that align with an organization's long-term goals and meet market needs effectively.
Q6: Place the following in order of increasing
Q46: Determine the volume of O<sub>2</sub> (at STP)
Q56: Determine the oxidizing agent in the following
Q63: It is possible to determine the ionization
Q81: Identify the major greenhouse gas.<br>A) CO<sub>2</sub><br>B) O<sub>2</sub><br>C)
Q120: Calculate the work, w, gained or lost
Q122: Describe a covalent bond.
Q158: The volume of a gas is proportional
Q171: Automotive air bags inflate when sodium azide
Q235: Lithium and nitrogen react to produce lithium