According to the following reaction, how much energy is required to decompose 55.0 kg of Fe3O4? The molar mass of Fe3O4 is 231.55 g 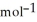 . Fe3O4(s) → 3Fe(s) + 2O2(g)
. Fe3O4(s) → 3Fe(s) + 2O2(g)  H° = +1118 kJ
H° = +1118 kJ
Definitions:
Chi-Square Value
A statistical measure used in tests of independence and goodness of fit, indicating how expectations compare to actual observed data.
Degrees of Freedom
Degrees of freedom refer to the number of independent values or quantities that can vary in an analysis without breaking any constraints.
Contingency Table
A data table that displays the frequency distribution of the variables.
Expected Frequency
In statistics, the predicted count of occurrences in a category of a contingency table based on probabilities derived from a theoretical distribution.
Q1: Use the periodic table to determine the
Q29: Identify the set of four quantum numbers
Q30: Identify the ground-state electron configuration for Sr.<br>A)
Q33: An unknown ideal gas was added to
Q50: Identify the numbers for m<sub>l</sub> for a
Q97: What are the possible values of l
Q97: Which reaction below represents the second ionization
Q110: How much energy (in kJ) do 3.0
Q134: Use the data given below to construct
Q243: Balance the following redox reaction if it