According to the following reaction, how much energy is evolved during the reaction of 2.50 L B2H6 and 5.65 L Cl2 (both gases are initially at STP) ? The molar mass of B2H6 is 27.67 g 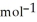 . B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g)
. B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g) 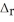 H° = -1396 kJ
H° = -1396 kJ
Definitions:
Women
Adult human females, often discussed in contexts of social, political, and economic rights and roles.
Muscle Strength
Muscle strength refers to the amount of force a muscle can produce with a single maximum effort.
Middle Adulthood
A stage of life typically defined by the ages of 40 to 65, where individuals experience several physical, psychological, and social changes.
Height
The measurement of someone or something from base to top or head to foot in a vertical manner.
Q4: Calculate the molecular weight of a gas
Q12: Place the following in order of increasing
Q36: How many moles of Co<sup>2+</sup> are present
Q37: Which of the following visible colours of
Q67: Explain why the lattice energy of MgS
Q70: 9.26 g of an unknown solid is
Q78: Give the temperature and pressure at STP.<br>A)
Q98: Give the number of lone pairs for
Q114: Choose the best Lewis structure for PO<sub>4</sub><sup>3</sup>⁻.<br>A)
Q235: Lithium and nitrogen react to produce lithium