Using the following equation for the combustion of octane, calculate the amount of moles of carbon dioxide formed from 100.0 g of octane. The molar mass of octane is 114.33 g  . The molar mass of carbon dioxide is 44.0095 g
. The molar mass of carbon dioxide is 44.0095 g 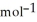 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 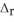 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Balance Sheet
A financial statement that shows a company's assets, liabilities, and shareholder equity at a specific point in time, offering a snapshot of its financial condition.
Stockholder
An individual or entity that owns shares in a corporation and therefore has a claim on part of its assets and earnings.
Share Price
The price of a single share of a company's stock in the stock market.
Consistent Dividends
Regular and predictable payments made by a company to its shareholders out of its profits.
Q32: valence electrons<br>A)electrons in the outermost shell<br>B)1<br>C)2<br>D)electrons in
Q52: A 4.98 g sample of aniline (C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,
Q57: Identify the ground-state electron configuration for Se<sup>2</sup>⁻.<br>A)
Q63: Which of the following is a precipitation
Q94: Choose the bond below that is the
Q118: How many millilitres of a 0.266 mol
Q121: How many moles of BCl<sub>3</sub> are needed
Q122: A sample of N<sub>2</sub> effuses in 255
Q197: Which of the following statements is TRUE?<br>A)
Q226: Lead ions can be precipitated from aqueous