Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 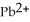 (aq) +
(aq) + 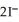 (aq) →
(aq) → 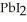 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion. How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of
(s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion. How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of 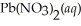 to completely precipitate the lead?
to completely precipitate the lead?
Definitions:
Preconventional Level
Refers to the first level of moral reasoning in Kohlberg's theory, characterized by understanding morality based on consequences and personal benefit.
Moral Development
The process by which individuals learn and apply moral codes, values, and understandings of right and wrong across their lifespan.
Pleasure
A feeling of happiness or satisfaction derived from an experience that is enjoyable or fulfilling.
Adoptive Families
Families that are formed through the legal process of adoption, where parents choose to take on the responsibility for raising a child as their own.
Q36: What group of elements in the periodic
Q40: When 1.50 mol of CH<sub>4</sub>(g) reacts with
Q80: What element is being oxidized in the
Q88: _ is a piece of equipment designed
Q93: Define constructive interference.
Q100: A 45.0 L steel tank at 20.0
Q114: Which of the following elements is classified
Q117: How would the concentration change if a
Q137: Consider a container of gas under a
Q183: How many chloride ions are there in