Two solutions, initially at 24.69 °C, are mixed in a coffee cup calorimeter (Ccal = 105.5 J °C-1) . When a 200.0 mL volume of 0.100 mol L-1 AgNO3 solution is mixed with a 100.0 mL sample of 0.100 mol L-1 NaCl solution, the temperature in the calorimeter rises to 25.16 °C. Determine the 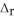 H°, in units of kJ
H°, in units of kJ 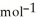 . Assume that the density and heat capacity of the solutions is the same as that of water. Hint: Write a balanced reaction for the process.
. Assume that the density and heat capacity of the solutions is the same as that of water. Hint: Write a balanced reaction for the process.
Definitions:
Orthopnea
Shortness of breath that occurs when lying flat, forcing the person to sleep propped up in bed or sitting in a chair.
Exacerbation
A worsening or increase in the severity of symptoms or disease.
Bronchovesicular Breath Sounds
Normal breath sounds heard over the main bronchus area and over the upper right posterior lung field, characterized by moderate pitch and intensity.
Pathologic Disease
A disease characterized by an identifiable set of signs or symptoms, often associated with disordered bodily function.
Q19: Using the graph below, determine the gas
Q70: A gas bottle contains 0. 350 mol
Q121: Identify what a bomb calorimeter measures.<br>A) measures
Q125: According to the following reaction, how much
Q128: Draw the Lewis structure for NO<sub>2</sub>⁻, including
Q135: How many unpaired electrons are there in
Q150: Identify the number of valence electrons for
Q156: What is the concentration (M) of a
Q157: Calculate the mass of a pickup truck
Q248: Identify the net ionic equation for the