According to the following reaction, how much energy is evolved during the reaction of 2.50 L B2H6 and 5.65 L Cl2 (both gases are initially at STP) ? The molar mass of B2H6 is 27.67 g 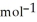 . B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g)
. B2H6(g) + 6Cl2(g) → 2BCl3(g) + 6HCl(g) 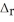 H° = -1396 kJ
H° = -1396 kJ
Definitions:
Altruism
Social interaction that has the potential to decrease the lifetime reproductive success of the member exhibiting the behavior.
Dominance Hierarchy
A social structure within a group of animals where individuals are ranked according to their level of dominance or power.
Pheromones
Chemical signals released by an organism that influence the behavior or physiology of other members of the same species.
Chemical Communication
The use of chemical signals between organisms, which can regulate behavior, identify individuals or species, and signal threats or resources.
Q35: The water at the top of a
Q40: When 1.50 mol of CH<sub>4</sub>(g) reacts with
Q45: Identify the compound with the lowest magnitude
Q72: A piece of gold with a mass
Q100: Use the information provided to determine <img
Q105: Calculate the final temperature of a 63.7
Q130: How many grams of calcium hydride are
Q168: Which of the following statements is FALSE?<br>A)
Q226: Lead ions can be precipitated from aqueous
Q236: Identify the balanced equation to show the