Multiple Choice
Use the 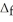 H° and
H° and 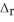 H° information provided to calculate
H° information provided to calculate 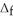 H° for SO3(g) :
H° for SO3(g) : 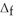 H° (kJ
H° (kJ 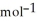 ) 2SO2(g) + O2(g) → 2SO3(g)
) 2SO2(g) + O2(g) → 2SO3(g) 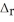 H° = -198 kJ SO2(g) -297
H° = -198 kJ SO2(g) -297
Definitions:
Related Questions
Q8: A sample of copper absorbs 43.6 kJ
Q13: Determine the limiting reactant (LR) and the
Q17: Place the following in order of decreasing
Q17: What is the concentration (M) of CH<sub>3</sub>OH
Q60: Using periodic trends, place the following bonds
Q84: Which of the following statements is TRUE?<br>A)
Q100: What was the predicted formula for the
Q124: According to the following reaction, how much
Q140: What pressure will 2.6 × 10<sup>23</sup> molecules
Q171: Automotive air bags inflate when sodium azide