Use the information provided to determine 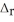 H° for the following reaction:
H° for the following reaction: 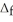 H° (kJ
H° (kJ 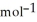 ) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g)
) 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g) 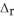 H° = ? Fe2O3(s) -824
H° = ? Fe2O3(s) -824
Fe3O4(s) -1118
CO(g) -111
CO2(g) -394
Definitions:
Debris
Scattered fragments, typically of something wrecked or destroyed.
Statute Of Frauds
State-level legislation that addresses the enforceability of contracts that fail to meet the requirements set forth in the statute; serves to protect promisors from poorly considered oral contracts by requiring certain contracts to be in writing.
Parol Evidence Rule
A principle in contract law that prevents the parties from presenting external evidence that contradicts or adds to the written terms of a contract.
Merger Clause
A contract provision stating that the written contract represents the complete and final agreement between the parties.
Q4: What is the oxidation number of the
Q9: Which of the following gases has the
Q23: Calculate the internal energy change, Δ<sub>r</sub>U, for
Q29: Define hypoxia.<br>A) oxygen starvation<br>B) increased oxygen concentration
Q29: An unknown metal alloy, specific heat capacity
Q56: Define effusion.
Q99: Give the electron configuration for O.<br>A) 1s<sup>2</sup>2s<sup>2</sup>2p<sup>4</sup><br>B)
Q109: Using the periodic table, identify the element
Q109: What is the maximum number of s
Q130: Each of the following sets of quantum