The combustion of titanium with oxygen produces titanium dioxide: Ti(s) + 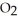 (g) →
(g) → 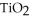 (s)
(s)
When 2.090 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °C to 91.30 °C. In a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kJ 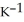 . The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ
. The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ 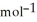 .
.
Definitions:
Patient Communication
The exchange of information between healthcare providers and patients, essential for effective diagnosis, treatment, and patient satisfaction.
Health History Form
A document used in medical settings to collect a patient's personal and familial health information, aiding in diagnosis and treatment planning.
Administrative Sheet
is a document used in healthcare settings to track the administration of medications and treatments to patients, ensuring accuracy and compliance with a prescribed care plan.
Personal Data
Information relating to an identified or identifiable person.
Q1: Use the information provided to determine <img
Q9: In the best Lewis structure for CN<sup>-</sup>,
Q52: What volume would 1.02 moles of Kr
Q65: Why does the size of the transition
Q71: If the percent yield for the following
Q81: Identify a characteristic of halogens.<br>A) powerful reducing
Q117: Ca-O<br>A)strongest covalent bond<br>B)longest covalent bond<br>C)weakest ionic bond<br>D)metallic
Q148: Define heat capacity.<br>A) the quantity of heat
Q154: Calculate the final orbital, n<sub>f</sub>, of an
Q154: Which of the following samples will have