In the presence of excess oxygen, methane gas burns in a constant pressure system to yield carbon dioxide and water: 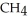 (g) +
(g) + 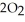 (g) →
(g) → 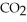 (g) +
(g) + 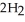 O (l)
O (l) 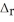 H = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
H = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
Definitions:
Projects
Planned tasks or activities that require effort and organization to achieve specific goals.
Frustrated
Feeling upset or annoyed, often due to inability to change or achieve something, leading to a sense of dissatisfaction and helplessness.
Home-Learning Activity
Educational tasks or projects assigned to students to be completed outside of school hours, often with the aim of reinforcing classroom learning.
Homework
Assignments given to students by their teachers to be completed outside the classroom.
Q60: How many grams of NaOH (MW =
Q89: A mixture of N<sub>2</sub>, O<sub>2</sub>, and Ar
Q97: Which reaction below represents the second ionization
Q108: Identify the oxidation state of Mg in
Q111: A syringe contains 0.65 moles of He
Q111: Choose the best Lewis structure for SF<sub>4</sub>.<br>A)
Q112: Using the following equation for the combustion
Q127: The volume of a gas is inversely
Q164: Identify the charge, X, on the phosphide
Q195: Identify the percent yield when 28.16 g