Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) +  (g) →
(g) → 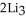 N(s)
N(s)
In a particular experiment, 5.50 g samples of each reagent are reacted. What is the theoretical yield of lithium nitride?
Definitions:
Variable Costing
An accounting method that only includes variable production costs (materials, labor, and overhead) in product cost calculations, excluding fixed costs.
Operating Income
Profit generated from a company's regular business operations, excluding expenses like taxes and interest.
Contribution Margin
The amount of revenue remaining after deducting variable costs, which can be used to cover fixed costs and contribute to profit.
Fixed Costs
Expenses that do not change with the level of output or sales over a short period, such as rent, salaries, and insurance.
Q5: Which element has the chemical symbol Ru?<br>A)
Q10: Use the standard reaction enthalpies given below
Q57: Which of the following pairs of aqueous
Q86: Which of the following statements is FALSE?<br>A)
Q89: How many anions are there in 2.50
Q99: Give the name of the element whose
Q119: Identify the name for aqueous H<sub>2</sub>SO<sub>4</sub>.<br>A) sulfuric
Q130: When 2.50 g of Ba(s) is added
Q183: How many moles of oxygen are formed
Q212: How many grams of AgNO<sub>3</sub> are needed