The half- reaction occurring at the anode in the balanced reaction shown below is . 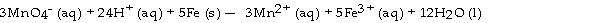
Definitions:
Markup
The uplift in the purchase price of items specifically to recover operational overhead and to make a profit.
Split-off Point
The stage in a production process where multiple products are derived from a single process or material, leading to separate further processing.
Joint Production Costs
Costs incurred in the process of producing two or more products simultaneously from the same raw materials or process.
Split-off Point
Refers to the stage in a production process where joint products can be recognized as separate products.
Q1: The value of ΔG° at 25 °C
Q1: The mole fraction of neon in dry
Q6: Roasting HgS in the presence of oxygen
Q8: Using Table 20.1, which substance can oxidize
Q22: The lead- containing reactant(s) consumed during recharging
Q25: Silver has two naturally occurring isotopes with
Q55: The town of Natrium, West Virginia, derives
Q62: If a metal forms more than one
Q87: Chromium is in the +6 oxidation state
Q91: Replacement of Na<sub>2</sub>O by K<sub>2</sub>O in soda-