Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 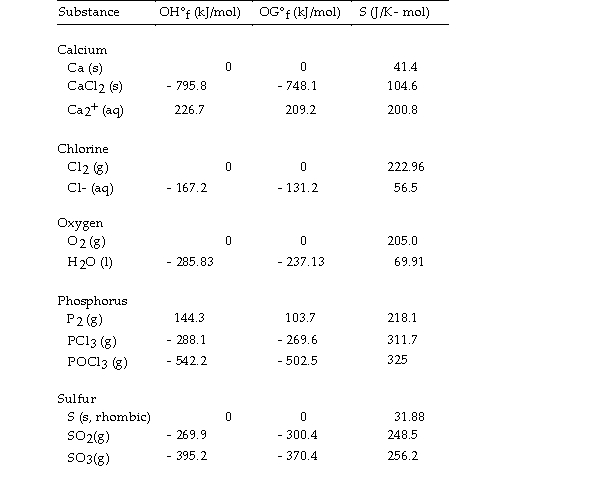
-The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
External Locus
A belief that control over one's life events and outcomes resides primarily with external forces or circumstances.
Julian Rotter
A psychologist known for his work in social learning theory and the development of the concept of locus of control.
Locus of Control
A psychological concept that refers to people's beliefs about the extent of control they have over events in their lives.
Reinforcement Value for Good Health
The perceived benefits or rewards that individuals associate with engaging in health-promoting behaviors.
Q3: Which below best describe(s) the behavior of
Q16: A 25.0 mL sample of a solution
Q38: When the cell potential is negative in
Q56: The correct name for N<sub>2</sub>O<sub>5</sub><sub> </sub>is _
Q72: The concentration of water vapor in a
Q81: The value of ΔG° at 373 °K
Q93: The rate of a second order reaction
Q99: How many moles of B are present
Q139: Which pair of elements would you expect
Q186: The formula for the compound formed between