Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 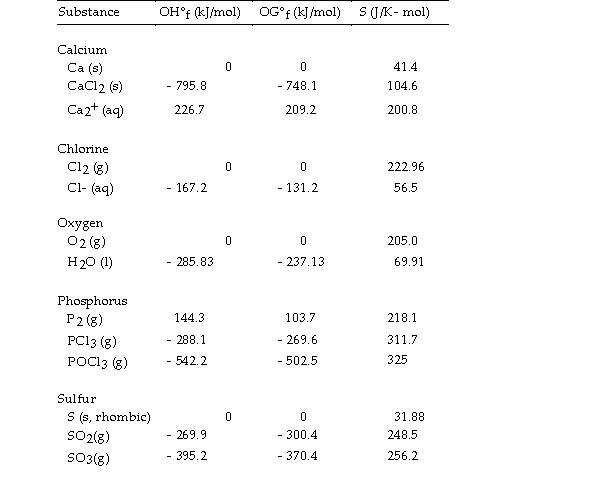
-The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
Definitions:
Milgram Experiment
A psychological experiment conducted by Stanley Milgram in the 1960s to study obedience to authority, where participants were instructed to administer electric shocks to another person.
Stanford University Prison Experiment
A psychological study conducted by Philip Zimbardo in 1971 at Stanford University, where students were assigned roles of prisoners and guards to explore the effects of perceived power.
Generalization
Drawing a conclusion about a certain characteristic of a population based on a sample from it.
Logical Support
The provision of reasons or evidence to justify a claim or argument.
Q21: Consider a pure crystalline solid that is
Q22: The relationship between the concentrations of reactants
Q48: The charge on the ion is -
Q50: Which one of the following molecular formulas
Q55: At 22 °C, K<sub>p </sub>= 0.070 for
Q62: Municipal water treatment consists of five steps
Q69: Ozone is a(n) of oxygen.<br>A) isotope<br>B) isomer<br>C)
Q73: The pressure of the atmosphere .<br>A) increases
Q84: The correct name for Mg(ClO<sub>3</sub>)<sub>2</sub><sub> </sub>is _
Q88: What is meant by the statement that