Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 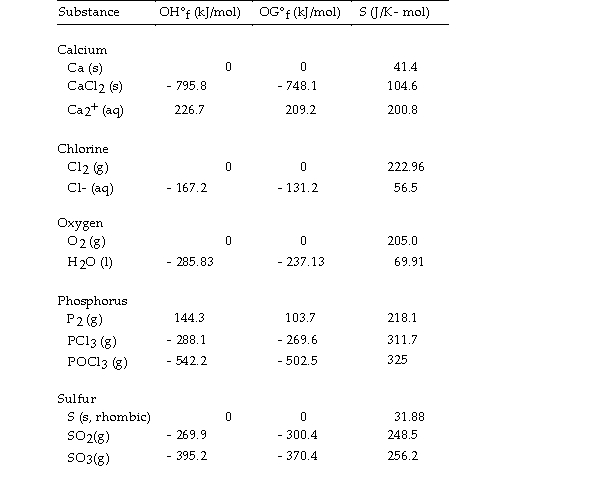
-The value of ΔG° at 25 °C for the formation of phosphorous trichloride from its constituent elements, 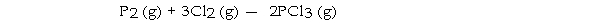 is kJ/mol.
is kJ/mol.
Definitions:
Ethical Problem
A dilemma or situation that requires an individual or organization to choose between alternatives that must be evaluated as right (ethical) or wrong (unethical).
Q10: Predict the charge of the most stable
Q16: The more the value of E°<sub>red</sub>, the
Q21: A solution is prepared by adding 1.43
Q41: Pure and pure are excluded from equilibrium-
Q42: A burning splint will burn more vigorously
Q48: A Lewis acid is an electron- pair
Q81: Which compound listed below has the smallest
Q90: The first law of thermodynamics can be
Q104: The rate of disappearance of HBr in
Q168: Which element forms an ion with the