Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 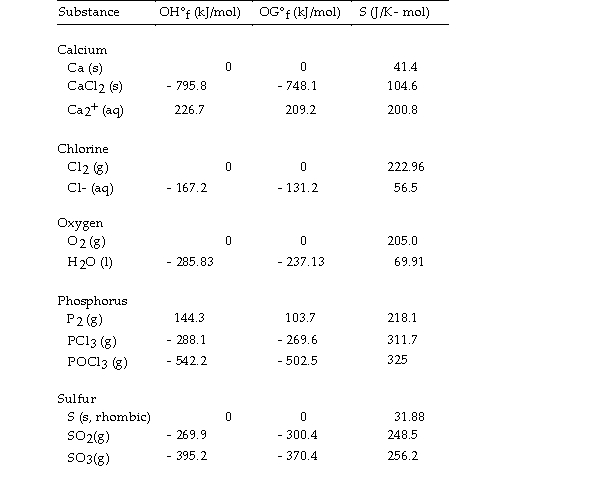
-The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
Simplifying Jobs
The act of making occupational tasks less complex, increasing efficiency and reducing the likelihood of errors.
Information-processing Requirements
The cognitive and mental demands placed on individuals to receive, process, store, and retrieve information.
Occupational Safety
refers to practices and policies in place to ensure the health and safety of employees in the workplace.
Ergonomic Job Design
The creation of work tasks, environments, and equipment to fit employee needs, aiming to increase efficiency and reduce discomfort or injury.
Q15: The element X has three naturally occurring
Q33: The mass of a proton is 1.00728
Q34: Calculate the percent ionization of formic acid
Q41: The world's largest desalinization plant is in
Q53: are used in automotive catalytic converters.<br>A) Noble
Q58: Find the temperature above which a reaction
Q71: CO<sub>2</sub><sub> </sub>from hydrocarbon combustion creates a major
Q80: Of the following processes, which one changes
Q97: Carbon- 11, fluorine- 18, oxygen- 15 and
Q123: Magnesium reacts with a certain element to