Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 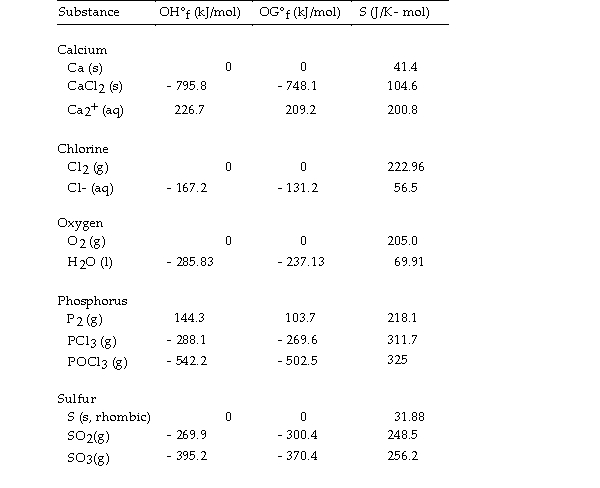
-The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is J/K.
is J/K.
Definitions:
Overall Trend
The general direction in which something is developing or changing over a period.
Exponentially
Describes a rapid increase in which something grows or changes at a steadily accelerating rate relative to its current value.
Water Purchases
The act of buying water or water rights, often in regions facing scarcity or for agricultural usage.
Government
The organization or system through which a community or nation is controlled and regulated.
Q3: The value of ΔG° at 25 °C
Q6: All atoms of a given element have
Q12: The use of radioisotopes in tracing metabolism
Q34: As one gains altitude in the atmosphere,
Q43: Which substance in the reaction below either
Q44: The concentration of S<sub>2</sub>O<sub>8</sub><sup>2</sup><sup>- </sup>remaining at 800
Q49: In the periodic table, the rows are
Q69: The value of ΔS° for the decomposition
Q92: A solution is prepared by dissolving calcium
Q110: Units of the rate constant of a