Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 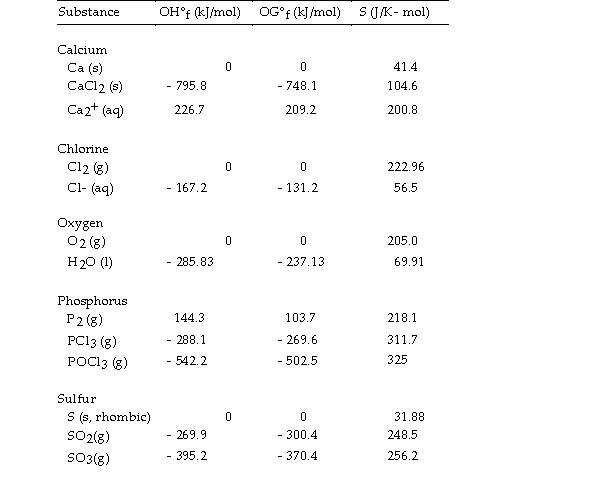
-The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is J/K.
is J/K.
Definitions:
Rapid Growth
Rapid Growth refers to a significant and faster-than-average increase in the size, revenue, or market share of a company or economy.
Economy
The large system that encompasses all production, exchange, distribution, and consumption of goods and services within a certain area. It involves activities and interactions among individuals, businesses, and governments.
Minimum-Variance Portfolio
A portfolio constructed to achieve the lowest possible risk (variance) for a given level of expected return, optimizing the risk-return tradeoff.
Expected Return
A financial term referring to the average amount of profit or loss an investment is predicted to generate based on historical data.
Q8: Nitric oxide arises from internal combustion engines.
Q8: A reaction vessel is charged with hydrogen
Q10: The value of K<sub>eq </sub>for the equilibrium<br>H<sub>2
Q29: What is the pH of an aqueous
Q36: The most useful ore of aluminum is
Q67: Predict the charge of the most stable
Q67: Calculate OG<sup>○</sup>(in kJ/mol) for the following reaction
Q69: The value of ΔS° for the decomposition
Q93: The pH of a 0.15 M aqueous
Q95: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt=" What is the