Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 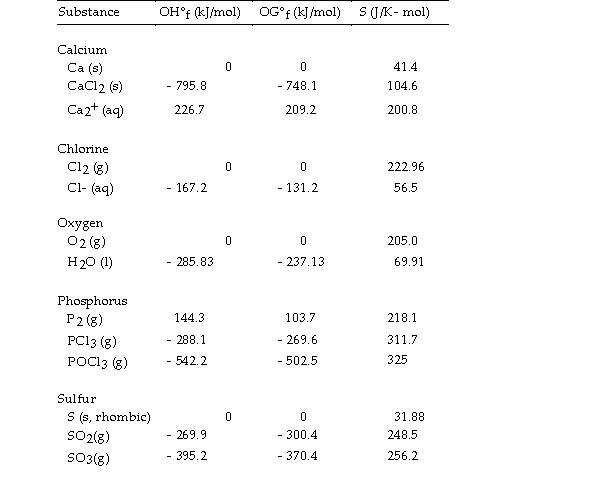
-The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
Definitions:
Stressor
A factor or event that causes stress to an organism, triggering a physical or emotional response.
Stress Reaction
The body's response to any demand or threat, involving physiological and psychological adjustments.
Lymphocyte
A type of white blood cell that is part of the immune system, helping the body to fight infections and diseases.
Macrophage
A type of white blood cell that engulfs and digests cellular debris, foreign substances, microbes, and cancer cells in a process called phagocytosis.
Q20: The half- life of a first- order
Q23: The half- life of a radionuclide<br>A) gets
Q34: The three radioactive series that occur in
Q42: One of the differences between a voltaic
Q51: _discovered radioactivity.
Q53: How is the reaction quotient used to
Q57: Nitrogen fixation is a difficult process because
Q67: Which one of the following graphs shows
Q74: The concentration of reactants or products at
Q108: Of the following, all are valid units