Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 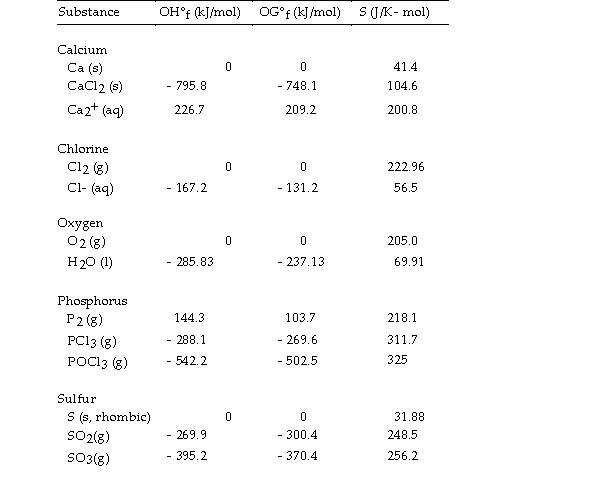
-The value of ΔH° for the decomposition of POCl3 into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
Related Questions
Q10: The value of ΔS° for the reaction<br>2C
Q34: The three radioactive series that occur in
Q36: Which atom has the smallest number of
Q39: The instability of xenon fluorides is due
Q50: In the reactions involved in the photodecomposition
Q75: The formula of a salt is XCl<sub>2</sub>.
Q89: Of the following acids, is not a
Q101: The reaction<br>CH<sub>3</sub>- N≡C - CH<sub>3</sub>- C≡N<br>Is a
Q134: Which type of formula provides the most
Q190: The correct name for Na<sub>3</sub>N is sodium