Multiple Choice
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 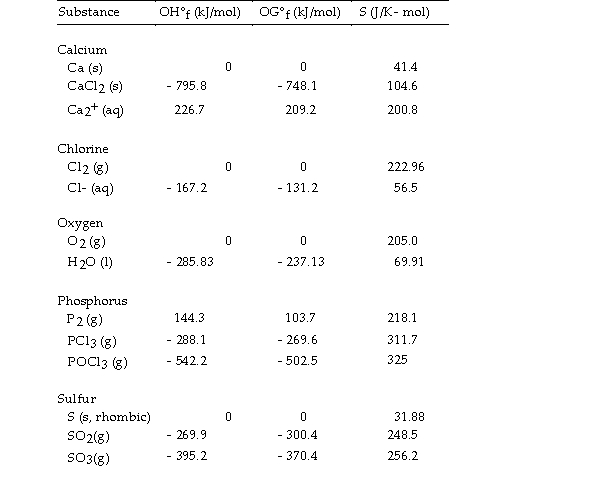
-The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
Comprehend the process and significance of aging on sensory and motor functions, including vision and reaction time.
Identify the definitions and examples of basic motor skills and reflexes in infancy.
Recognize the impact of aging on physical health, including common diseases and the concept of physiological age.
Understand the role of genetics, environment, and lifestyle on the development of motor skills and sensory functions across the lifespan.
Definitions:
Related Questions
Q4: The formula for aluminum hydroxide is .<br>A)
Q33: Which one of the following processes produces
Q39: Nitrogen dioxide decomposes to nitric oxide and
Q42: The lime- soda process is used for
Q49: The source(s) of sulfur dioxide in the
Q81: The value of ΔG° at 373 °K
Q86: Suppose you have just added 200.0 ml
Q106: The correct name for HClO<sub>2</sub><sub> </sub>is _
Q156: Of the following, contains the greatest number
Q192: Elements in Group 2A are known as