Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 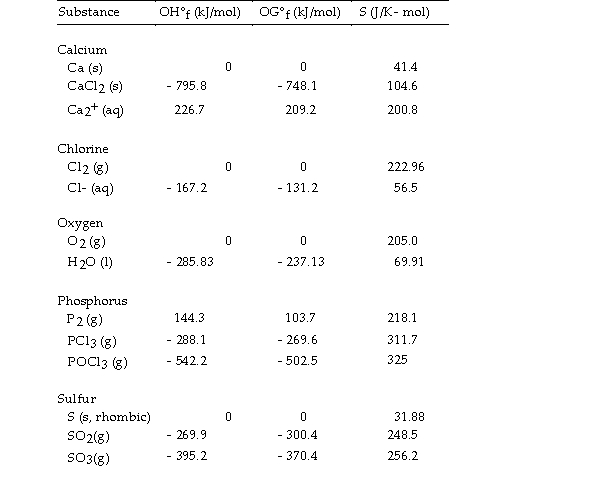
-The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is kJ/mol.
is kJ/mol.
Definitions:
Lender Fraud
Illegal practices committed by a lender, including deceptive actions to take advantage of a borrower or to approve a loan under false pretenses.
Premium On Bonds Payable
The amount by which a bond's selling price exceeds its face value, reflecting higher market interest rates at the time of issuance.
Maturity Value
The total amount payable to an investor at the end of a fixed term investment, including the principal and any accrued interest.
Q1: A 25.0 mL sample of an HCl
Q45: The active site of nitrogenase is a
Q47: Calculate the pH of a solution prepared
Q50: Which one of the following molecular formulas
Q50: The value of K<sub>eq </sub>for the following
Q60: The layer of the atmosphere that contains
Q62: The major type of cancer caused by
Q75: The solubility of a slightly soluble salt
Q77: The value of ΔG° at 373 K
Q93: The pH of a 0.15 M aqueous