Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 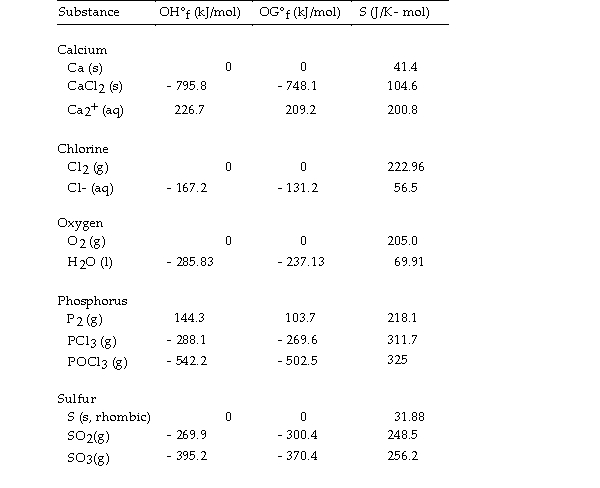
-The equilibrium constant for a reaction is 0.48 at 25 °C. What is the value of ΔG° (kJ/mol) at this temperature?
Definitions:
Rule of Thumb for Advertising
Simplified principles or guidelines that provide a baseline for allocating advertising budgets or measuring advertising effectiveness.
Price Elasticity of Demand
An indicator of the responsiveness of demand for a product to variations in its price, demonstrating how sensitive the quantity of the product demanded is to price fluctuations.
Advertising Elasticity of Demand
A measure of how advertising expenditures influence the quantity demanded of a product or service.
Price Elasticity of Demand
Measures how much the quantity demanded of a good responds to a change in its price, with higher elasticity indicating greater sensitivity to price changes.
Q11: The equilibrium- constant expression depends on the
Q24: SO<sub>2</sub>Cl<sub>2</sub><sub> </sub>decomposes in the gas phase by
Q29: The value of ΔH° for the formation
Q34: Calculate the percent ionization of formic acid
Q44: The standard emf for the cell using
Q47: A particular first- order reaction has a
Q48: The combustion of ethane in the presence
Q54: When two atoms of <sup>2</sup>H are fused
Q64: The quantity of charge passing a point
Q65: Approximately 90% of the earth's ozone is