Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 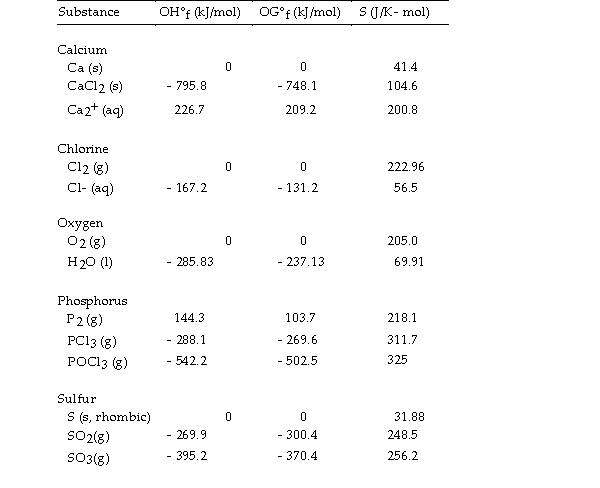
-The value of ΔG° at 25 °C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen,  is kJ/mol.
is kJ/mol.
Definitions:
Intrinsic Motivation
The drive to engage in an activity for its own sake, without the influence of external rewards or pressures.
Extrinsic Motivation
Motivation driven by external rewards such as money, fame, grades, or praise.
Self-efficacy
Someone's trust in their own skill to perform tasks needed for certain achievements.
Competence
The ability to do something successfully or efficiently, often referring to a specific skill set or knowledge area.
Q6: The formula of bromic acid is _.<br>A)
Q14: The value of OS° for the formation
Q22: The relationship between the concentrations of reactants
Q27: In what year was Fritz Haber awarded
Q33: Which one of the following statements regarding
Q43: Consider the following equilibrium.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt="Consider the
Q72: Carbon- 11 decays by positron emission:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg"
Q88: The major product of a hydrogen fuel
Q94: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt="For the
Q103: How much energy is produced when 0.082