A substance has a melting point of 20°C and a heat of fusion of 3.9 × 104 J/kg. The boiling point is 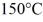 and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K) , 1000 J/(kg∙K) , and 400 J/(kg∙K) , respectively. The quantity of heat required to raise the temperature of
and the heat of vaporization is 7.8 × 104 J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg∙K) , 1000 J/(kg∙K) , and 400 J/(kg∙K) , respectively. The quantity of heat required to raise the temperature of 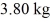 of the substance from
of the substance from 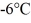 to
to 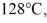 at a pressure of 1.0 atm, is closest to
at a pressure of 1.0 atm, is closest to
Definitions:
Adolescents
Individuals in the transitional stage of physical and psychological development that generally occurs during the period from puberty to legal adulthood.
Preconscious
Refers to the thoughts, feelings, and memories that are not in immediate conscious awareness but can be easily accessed.
Superego
A component of Freud's psychoanalytic theory, representing the moralistic part of the mind that incorporates social standards and ideals.
Ego-ideal
A component of the psyche that represents an individual's standards of perfection and aspirations.
Q17: A proton, with mass 1.67 × 10<sup>-27</sup>
Q18: A tire is rolling along a road,
Q20: During each cycle of operation, a refrigerator
Q21: A sealed container holds 0.020 moles of
Q22: Two long parallel wires placed side-by-side on
Q46: The figure shows the displacement y of
Q49: A satellite that weighs 4900 N on
Q49: A charge of 2.00 μC flows onto
Q62: A torque of 12 N ∙ m
Q84: A 5.0-m long, 12-kg uniform ladder rests