A sealed 89- 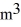 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The initial pressure of the gas is closest to
Definitions:
Interest Rate
The percentage of a sum of money charged for its use, typically expressed as an annual percent rate.
Bank Deposits
Funds that are placed into banking institutions for safekeeping, which can include savings accounts, checking accounts, and certificates of deposit.
Loans
Money lent to individuals or entities with the expectation of repayment within a specific timeframe, usually with interest.
Default
The failure to meet the legal obligations or conditions of a loan, such as not making the scheduled payments.
Q7: Suppose a region of space has a
Q15: If the current density in a wire
Q22: 1.000 L of water at 20.00°C will
Q29: The process shown in the T-V diagram
Q32: A stationary siren emits sound of frequency
Q35: A solid steel sphere with a radius
Q37: A satellite is orbiting the earth. If
Q41: A carousel that is 5.00 m in
Q77: An 82.0 kg-diver stands at the edge
Q107: A figure skater rotating at 5.00 rad/s