What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol ∙ K = 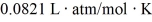 .
.
Definitions:
Midwife
A healthcare professional specializing in supporting women during childbirth, providing prenatal and postpartum care.
Doula
A trained professional who provides continuous physical, emotional, and informational support to a mother before, during, and shortly after childbirth.
Competent Parents
Individuals who possess the skills, knowledge, and emotional capacity to effectively raise and provide for their children.
Developmental Needs
The necessary conditions or stimuli required to support the natural progression of human growth and development at different life stages.
Q13: A 2.00-m rod of negligible mass connects
Q21: Which statements are true for an electron
Q22: The cross section of a long coaxial
Q22: A point charge Q = -500 nC
Q25: The second law of thermodynamics leads us
Q41: The plot in the figure shows the
Q49: The figure shows a pV diagram for
Q49: Tensile strain is<br>A) the ratio of the
Q52: A parallel-plate capacitor has a capacitance of
Q85: A cylindrical bar that us well insulated