One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO₃) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO₃⁻) and a hydrogen ion (H⁺) . (See figure.) 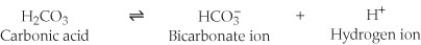
If the pH of blood drops, one would expect ________.
Definitions:
Christmas Cards
Greetings exchanged during the Christmas season, often featuring holiday themes and expressions of goodwill.
Sociology Professor
An academic expert who specializes in the study of society, social relationships, and social behavior, typically teaching and conducting research at a university.
Significant Minority
A portion of a group that is not the majority but large enough to have an impact or warrant attention.
Gratitude
A positive emotion that results from the perception that one has benefited from the costly, intentional, voluntary action of another person.
Q8: You would like to lose weight. Which
Q15: The difference between an aldose sugar and
Q19: Oxygen has an atomic number of 8
Q32: Which is the first step to be
Q37: A controlled experiment is one that<br>A) proceeds
Q47: The relationship between catabolism and anabolism is
Q47: What kinds of molecules pass through a
Q51: Kelly is the chief marketing officer of
Q52: An organism with a cell wall would
Q55: Roler Telecom,a leading mobile telephone operator in