The following figure illustrates the action of a HF and F buffer where the sizes of the boxes are proportional to the concentrations of HF and F in solution. Which of the following chemical equations represents the reaction that occurs when -OH is added to the HF/F buffer? 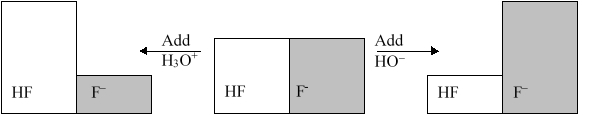
Definitions:
Limited Partner
An investor in a partnership whose liability is limited to the amount of their investment, not involved in day-to-day management.
Management Powers
The authority vested in the directors, officers, or managers of an organization to make decisions and conduct the operations of the business.
Uniform Limited Partnership Act
A set of laws adopted by some states that govern the formation, operation, and dissolution of limited partnerships.
Admission Condition
Requirements that must be met or agreed upon before an individual can be accepted or admitted into a program, organization, or agreement.
Q2: Mandy is a 40% partner in The
Q10: Theodore is 37 years old. He earned
Q43: Which of the hydrocarbons listed are unsaturated?
Q65: Which of the following molecules contains a
Q70: An electrolyte is a substance that<br>A) dissolves
Q92: In the acid-base reaction between ammonia and
Q98: The reaction that occurs in a hydrogen
Q104: According to the chart below, how are
Q105: Which of the following transformations is a
Q139: Edmiston Company reported the following year-end information: