Consider the Figure Below Which Shows G For a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G for a chemical process plotted against absolute temperature. 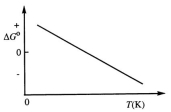 From this plot, it is reasonable to conclude that:
From this plot, it is reasonable to conclude that:
Definitions:
Mediate Dispute
The act of resolving a conflict or disagreement between two or more parties through the intervention of a neutral third party.
Company Policies
Are guidelines established by businesses to govern their operations, employee behavior, and company procedures.
Work Assignments
Tasks or projects allocated to employees or team members as part of their job responsibilities.
Participative Management
Participative Management is a management approach whereby employees at all levels are involved in the decision-making processes of the organization.
Q11: Fill in the blank spaces and write
Q12: Given: C<sub>2</sub>H<sub>2</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q13: Sodium oxide combines violently with water. Which
Q21: Which of the following isotopes is most
Q22: The following reaction, in CCl<sub>4</sub> solvent,
Q29: Which relationship or statement best describes
Q30: Who is credited with first measuring the
Q45: Butadiene, C<sub>4</sub>H<sub>6</sub> (used to make synthetic rubber
Q74: The iodine "clock reaction" involves the
Q84: Who is credited with discovering the atomic