Consider the Figure Below Which Shows G For a Chemical Process Plotted Against Absolute Temperature
Consider the figure below which shows G for a chemical process plotted against absolute temperature. 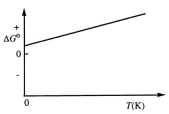 From this plot, it is reasonable to conclude that:
From this plot, it is reasonable to conclude that:
Definitions:
Classification
The action or process of categorizing something according to shared qualities or characteristics.
Organization
A structured group of individuals working together towards a common goal or set of goals, typically involving defined roles, responsibilities, and processes.
Conceptualization
The process of forming a concept or notion of something.
Concretization
The process of making abstract ideas or concepts more tangible and concrete, often through visual representation or practical examples.
Q18: What is the highest possible oxidation state
Q20: Predict the products of the cell reaction
Q26: The rate law for the reaction
Q32: What is the E <span class="ql-formula"
Q33: (p. Various sections) For a given
Q46: Calcium oxide is added to molten iron
Q61: If 10.0 g of NaF and 20.0
Q66: Modern studies have shown that the Law
Q94: Consider the reaction of iodine with
Q98: A solution is prepared by adding 100