Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. 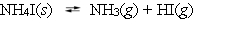
At 400 C, Kp = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400 C.
Definitions:
Marxist And Fordist
Concepts referring to the Marxist critique of capitalist industrial production and the Fordist model of mass production and labor standardization, respectively.
Social And Political
Relating to society and its organization or the theory and practice of politics and government.
Human Relations Theory
A management approach emphasizing the importance of social processes in the workplace and the well-being of employees in improving productivity.
Human Resources Management
The strategic approach to the effective management of people in an organization in a way that helps the business gain a competitive advantage.
Q44: Potassium nitrate is a strong reducing agent.
Q48: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q56: The formation constant for the reaction
Q65: In which of the following ways is
Q86: A diprotic acid H<sub>2</sub>A has K<sub>a1</sub> =
Q89: Predict the products for the reaction
Q90: The normal boiling point of ether is
Q93: Consider the dissolution of MnS in water
Q94: element that lies in period 4?<br>A) Cr<br>B)
Q109: The salts X(NO<sub>3</sub>)<sub>2</sub> and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>