The reaction of nitric oxide to form dinitrogen oxide and nitrogen dioxide is exothermic. 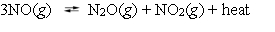 What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
What effect will be seen if the temperature of the system at equilibrium is raised by 25 C?
Definitions:
Acronym
A word created from the initial letters of a phrase, making it easier to recall or say, such as NASA from National Aeronautics and Space Administration.
Chunking
A process of grouping information into larger units to improve memory and learning.
Heuristic
A mental shortcut that allows people to solve problems and make judgments quickly but can sometimes lead to errors in thinking.
Acrostic
A poem, word puzzle, or other composition in which certain letters in each line form a word or words.
Q9: There is a direct correlation between the
Q39: Aqueous solutions of phosphoric acid and sodium
Q50: Increasing the concentrations of the components of
Q56: What is the pOH of a 0.0250
Q59: Barium fluoride is used in embalming and
Q60: . If the compositions of the two
Q75: At 850 <span class="ql-formula" data-value="\degree"><span
Q79: The salts X(NO<sub>3</sub>)<sub>2</sub> and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q93: The mole fraction of potassium nitrate in
Q115: When 0.300 g of a diprotic acid